5 steps lewis dot structure how to do draw lewis dot structure
Table of Contents
Table of Contents
If you’ve ever wondered how to draw a Lewis dot diagram, you’re not alone. For many students, chemistry can be a challenging subject, and Lewis dot diagrams are no exception. However, mastering this skill is essential for understanding chemical bonding and predicting the behavior of molecules. By the end of this post, you’ll have a solid grasp on how to draw a Lewis dot diagram and develop the confidence to tackle even the most complex molecules.
When it comes to drawing Lewis dot diagrams, the process can seem overwhelming at first. Knowing where to start and what rules to follow can be confusing, especially if you’re not familiar with the concept of valence electrons. Figuring out how to arrange electrons, count bonds, and determine lone pairs can often leave students feeling frustrated and lost. However, these pain points can be easily overcome with a bit of practice and guidance.
So, how do you draw a Lewis dot diagram? The first step is to determine the number of valence electrons in the atom or molecule you’re drawing. Next, arrange the electrons in pairs around the central atom, placing any remaining electrons as lone pairs. Finally, add one bond for each pair of electrons shared between two atoms. By following these steps, you can draw a Lewis dot diagram that accurately reflects the electron arrangement in a molecule or atom.
In summary, drawing Lewis dot diagrams requires a basic understanding of valence electrons and a few simple rules. By arranging electrons and counting bonds and lone pairs, you can represent the electron arrangement in any molecule or atom. With practice and patience, drawing Lewis dot diagrams will become second nature, and you’ll gain a deeper understanding of chemical bonding and molecular behavior.
How to Draw Lewis Dot Diagrams: A Personal Experience
When I first learned how to draw Lewis dot diagrams, I found the process confusing and overwhelming. As a visual learner, I had trouble conceptualizing the arrangement of electrons and understanding how they contributed to the overall structure of a molecule. However, with the help of a patient tutor and lots of practice problems, I eventually began to grasp the concept and gain confidence in my abilities.
One of the key things I learned was the importance of understanding the concept of valence electrons. Once I had a solid grasp on how these electrons could be arranged in pairs and lone pairs, the process of drawing Lewis dot diagrams became much clearer. Additionally, I found it helpful to work through practice problems step-by-step, starting with simple molecules and gradually increasing in complexity.
Tips for Drawing Lewis Dot Diagrams
When it comes to drawing Lewis dot diagrams, there are a few tips and tricks that can make the process easier. One helpful strategy is to identify the central atom first, then determine the number of valence electrons that atom possesses. Another useful approach is to work systematically, starting with the simplest molecules and gradually increasing in complexity. Finally, it’s important to remember that practice makes perfect - the more you work through practice problems, the more comfortable and confident you’ll become with drawing Lewis dot diagrams.
Valence Electrons and Bonding
Valence electrons play a crucial role in chemical bonding, and understanding their arrangement can help predict the behavior of molecules. In general, atoms with a full valence shell of electrons are more stable and less likely to react with other atoms. Additionally, the number of valence electrons in an atom determines its ability to form bonds with other atoms. For example, atoms with one to three valence electrons tend to lose those electrons and form positive ions, while atoms with five to seven valence electrons tend to gain electrons and form negative ions.
Bond Types in Lewis Dot Diagrams
When drawing Lewis dot diagrams, it’s important to understand the different types of bonds that can form between atoms. Covalent bonds, for example, occur when two atoms share electrons in order to fill their valence shells. Ionic bonds, on the other hand, occur when atoms transfer electrons between them, resulting in positively and negatively charged ions that attract each other. By understanding these bond types and their respective properties, you’ll be better equipped to draw accurate and informative Lewis dot diagrams.
The Importance of Drawing Lewis Dot Diagrams
While drawing Lewis dot diagrams can be challenging, it’s a crucial skill for understanding chemical bonding and predicting the behavior of molecules. By representing the electron arrangement in a molecule or atom, Lewis dot diagrams provide a visual representation of the bonding patterns that occur in nature. Additionally, they can be extremely useful tools for understanding the behavior of acids, bases, and other chemical compounds.
Question and Answer
1. What is the purpose of drawing a Lewis dot diagram?
The purpose of drawing a Lewis dot diagram is to visually represent the arrangement of electrons in a molecule or atom. By showing the bonding patterns between atoms, Lewis dot diagrams can help predict the behavior of chemical compounds and provide valuable insight into their properties and reactivity.
2. What are valence electrons?
Valence electrons are the outermost electrons in an atom, located in the highest electron shell. These electrons play an important role in chemical bonding because they are involved in forming chemical bonds with other atoms.
3. What are some common bond types in Lewis dot diagrams?
Some common bond types in Lewis dot diagrams include covalent bonds, ionic bonds, and metallic bonds. Covalent bonds occur when two atoms share electrons to fill their valence shells, while ionic bonds occur when atoms transfer electrons between them to form ions. Metallic bonds occur when electrons are shared between metal atoms in a lattice structure.
4. How can Lewis dot diagrams be used to predict the behavior of chemical compounds?
By showing the arrangement of electrons and bonding patterns in a molecule or atom, Lewis dot diagrams can help predict the behavior of chemical compounds. For example, they can help predict which compounds will be acidic or basic, or which compounds will interact to form new compounds or molecules.
Conclusion of How to Draw a Lewis Dot Diagram
Learning how to draw a Lewis dot diagram can be challenging, but it’s a crucial skill for understanding chemical bonding and predicting the behavior of molecules. By understanding the concept of valence electrons and bond types, and practicing with a variety of molecules, you’ll gain the confidence and knowledge to draw accurate and informative Lewis dot diagrams. So go forth and start practicing - your chemistry skills will thank you!
Gallery
A Simple Guide For Learning How To Draw Lewis Dot Structures. Use This

Photo Credit by: bing.com / lewis structure dot draw chemistry molecular structures infographic notes organic worksheet classroom help simple drawing diagram electron geometry worksheets chemical
【5 Steps】Lewis Dot Structure:How To Do|Draw Lewis Dot Structure | Diagram

Photo Credit by: bing.com / lewis structure dot
Lewis Structure Worksheet 1 Answer Key

Photo Credit by: bing.com / lewis electron rb rubidium
Mainly Science@Gisboyshigh
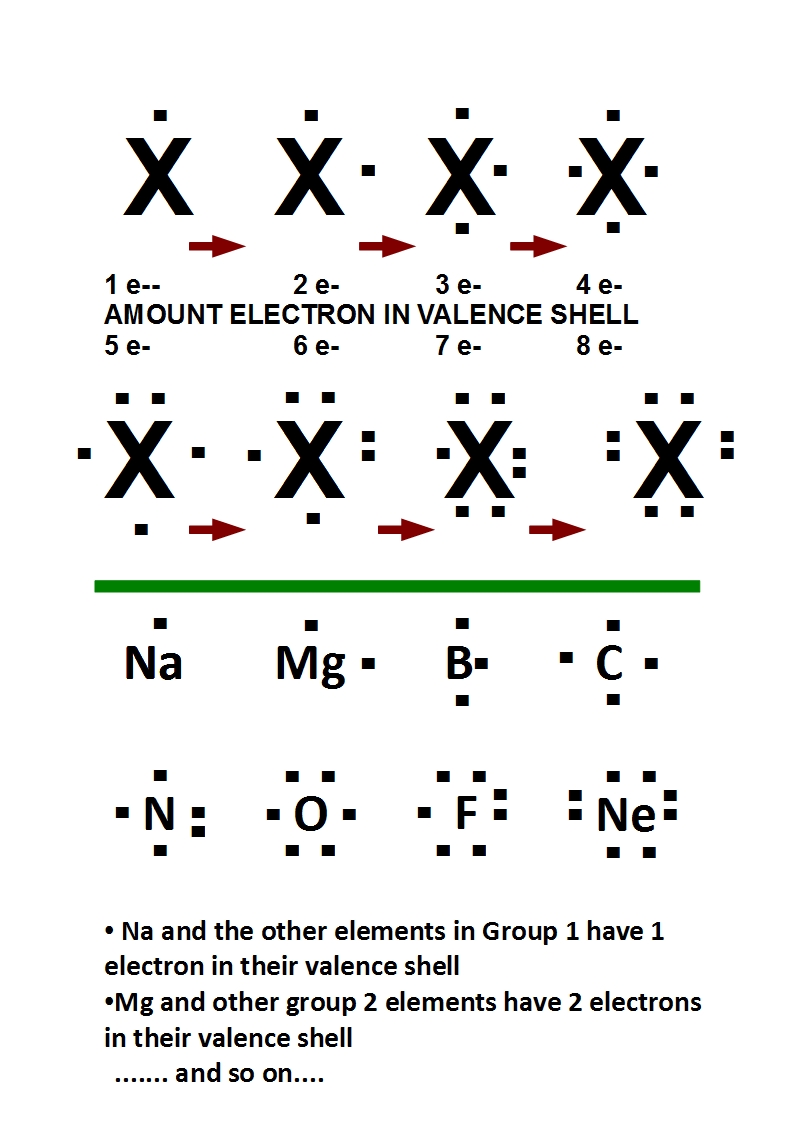
Photo Credit by: bing.com / dot lewis diagram beryllium structure elements diagrams electron labeled covalent atom science electrons chemistry compound calcium amazing wallpapers hd valence
Lewis Diagrams Made Easy: How To Draw Lewis Dot Structures - Watch

Photo Credit by: bing.com / jetindir
