How to draw energy level diagrams
Table of Contents
Table of Contents
If you’re studying chemistry, physics or material science, chances are you’ve come across energy diagrams. Energy diagrams are a visual representation that shows the energy levels of a system, such as the energy of molecules or electrons. If you’re wondering how to draw an energy diagram, keep reading to discover a step-by-step guide.
Understanding energy diagrams can be challenging, especially for beginners. One of the most common difficulties is knowing where to start and how to visualize the system’s energy levels. Another challenge is properly labeling the axes and interpreting the curves. Lastly, knowing how to use the energy diagram to predict the behavior of the system can also be a pain point.
To draw an energy diagram, you’ll need to follow these steps. First, identify the system whose energy levels you want to represent in the diagram. This could be anything from a molecule to a subatomic particle. Then, determine the energy levels of the system, and draw the corresponding y-axis. The x-axis represents the reaction coordinate, which could be anything that changes the energy of the system, such as temperature or pressure.
Next, plot the energy levels on the y-axis and connect the dots to create a curve. Depending on the complexity of the system, there could be multiple curves representing various transitions or states of the system. Finally, label the axes, add any necessary annotations such as the activation energy, and use the diagram to analyze the behavior of the system.
My Personal Experience with Energy Diagrams
When I took my first chemistry class in college, I struggled with energy diagrams. I found it hard to visualize the different energy levels and how they related to each other. After reading textbooks and practicing with diagrams, I finally understood how to draw energy diagrams. They became a useful tool for analyzing reactions, predicting behavior, and interpreting experiments.
How to Interpret Energy Diagrams
To interpret energy diagrams, it’s essential to understand how the curves relate to the system’s behavior. For example, the peaks in the curve represent the activation energy required for the system to transition from one state to another. The area under the curve represents the thermodynamic stability of the system. A flat curve at a low energy level implies the system is stable and not reactive, whereas a high-energy-level curve denotes an unstable or reactive system.
How to Use Energy Diagrams for Reaction Analysis
Energy diagrams are a useful tool for analyzing chemical reactions. By comparing the energy levels of the reactants and products, you can predict whether a reaction will be exothermic or endothermic, and determine the activation energy needed to proceed. The shape of the curve can also indicate whether the reaction will proceed as a one-step or multi-step process.
How to Draw Energy Diagrams in R
If you prefer to use R for data visualization, you can draw energy diagrams using the ggplot2 package. The library provides a geom_step function that allows you to plot energy levels and connect the dots. You can customize the curve’s color, shape, and size to your liking, add annotations and axis labels.
Conclusion of How to Draw an Energy Diagram
Drawing an energy diagram can be challenging, but by following the steps above and understanding the system’s behavior, you can create an accurate representation. Energy diagrams are a useful tool for chemistry, physics, and material science, providing insight into energy levels, transitions, and reactions.
Question and Answer
What is the purpose of an energy diagram?
An energy diagram visualizes the energy levels of a system, such as the energy of molecules or electrons. It can provide insight into the system’s behavior, such as transitions, stability, and reactions.
How do you draw an energy diagram?
To draw an energy diagram, you need to identify the system, determine energy levels, plot them on the y-axis, connect the dots to create curves, label the axes, and analyze the system’s behavior.
What is the activation energy in an energy diagram?
The activation energy is the energy required for a system to transition from one state to another. It is represented by the peaks in the curve and can provide insight into the reaction mechanism and rate.
What is an exothermic reaction?
An exothermic reaction is a chemical reaction that releases energy in the form of heat or light. The energy level of the products is lower than that of the reactants, resulting in a negative change in enthalpy.
Gallery
Energy Diagram — Overview & Parts - Expii

Photo Credit by: bing.com / monahan
How To Draw Energy Level Diagrams - YouTube
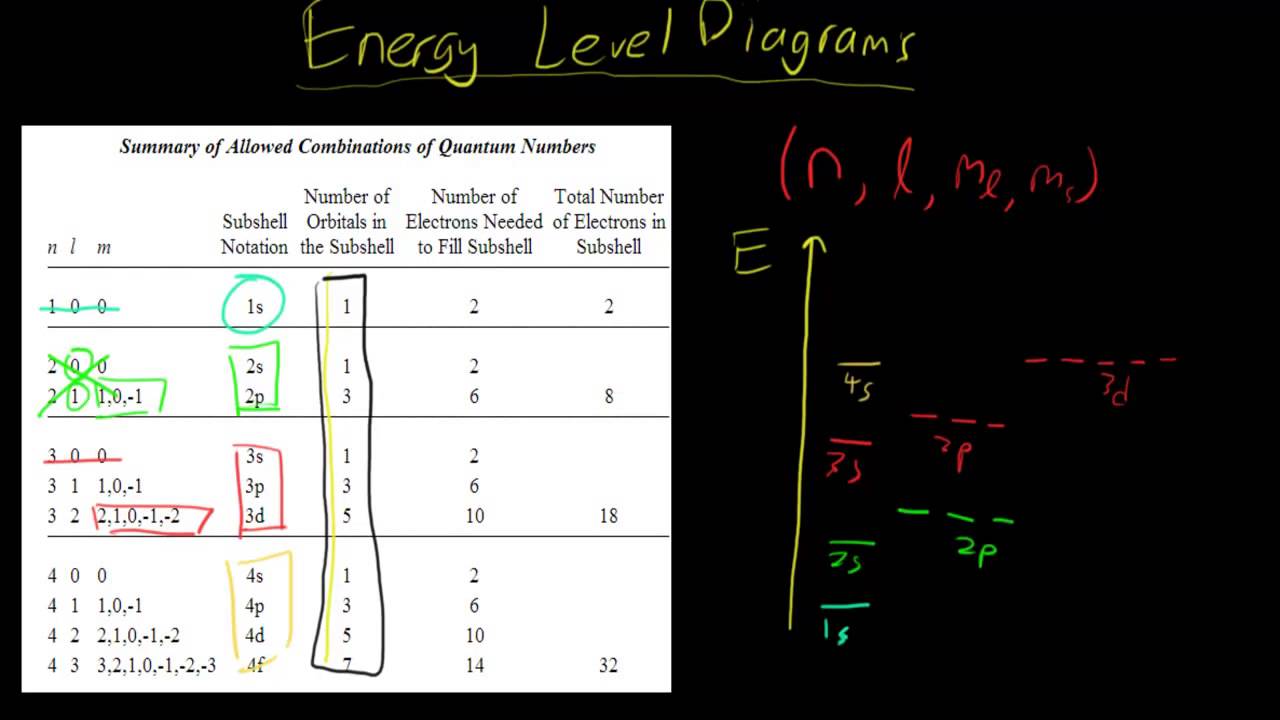
Photo Credit by: bing.com / energy level draw diagrams psx
How To Draw Energy Level Diagrams - YouTube

Photo Credit by: bing.com / energy level draw diagrams
How To Draw The Curves In An Energy Diagram In R? - Stack Overflow

Photo Credit by: bing.com / energy diagram reaction draw example curves chlorination wiring study following curve points would through look stack
Energy Diagram — Overview & Parts - Expii

Photo Credit by: bing.com / diagrams monahan